Exosomes are a hot commodity in regenerative medicine and aesthetic right now, and the market demand is pressuring small-scale production to pivot into mass production, and it is not an easy feat either. The challenge isn’t just in the science — but the precise control of every details and consistency in the process.
In exosome manufacturing, equipment selection and process setup play a crucial role in ensuring product quality, safety, and scalability. As exosome applications shift toward injectable- and clinical-grade products, the bar rises — from a common lab to a fully compliant pharmaceutical production site.
This article explores the essential equipment utilized in the exosome production process, from cell culture to aseptic filling and freeze-drying. See how integrated systems can help build a GMP-compliant, high-throughput aseptic production line.
How the Decision on Exosome Equipment Determine Product Quality and Scalability
Exosomes are fragile and temperature sensitive biological substance, and the production require precise control of environment and equipment reliability. For different products such as aesthetic products, raw materials for cell therapy, or clinical-grade therapeutics, the equipment the essential driver of product quality, safety, and scalability.
Poor equipment selections can undermine even the best source materials and formulations, by causing issues like contaminated filling, loss of bioactivity during freeze-drying, or unstable output that leads to failed batches and recalls. Investing in suitable aseptic systems and integrated solutions elevate your process from “adequate” into “future-proof sustainable production” — built with scalability and suitainability in mind.
More exosome manufacturers are shifting to strategic, end-to-end equipment planning — building a robust, compliant, and future-proof production line.
What Equipment Is Used in Exosome Manufacturing? A Fully Integrated Workflow from Cells to Final Product
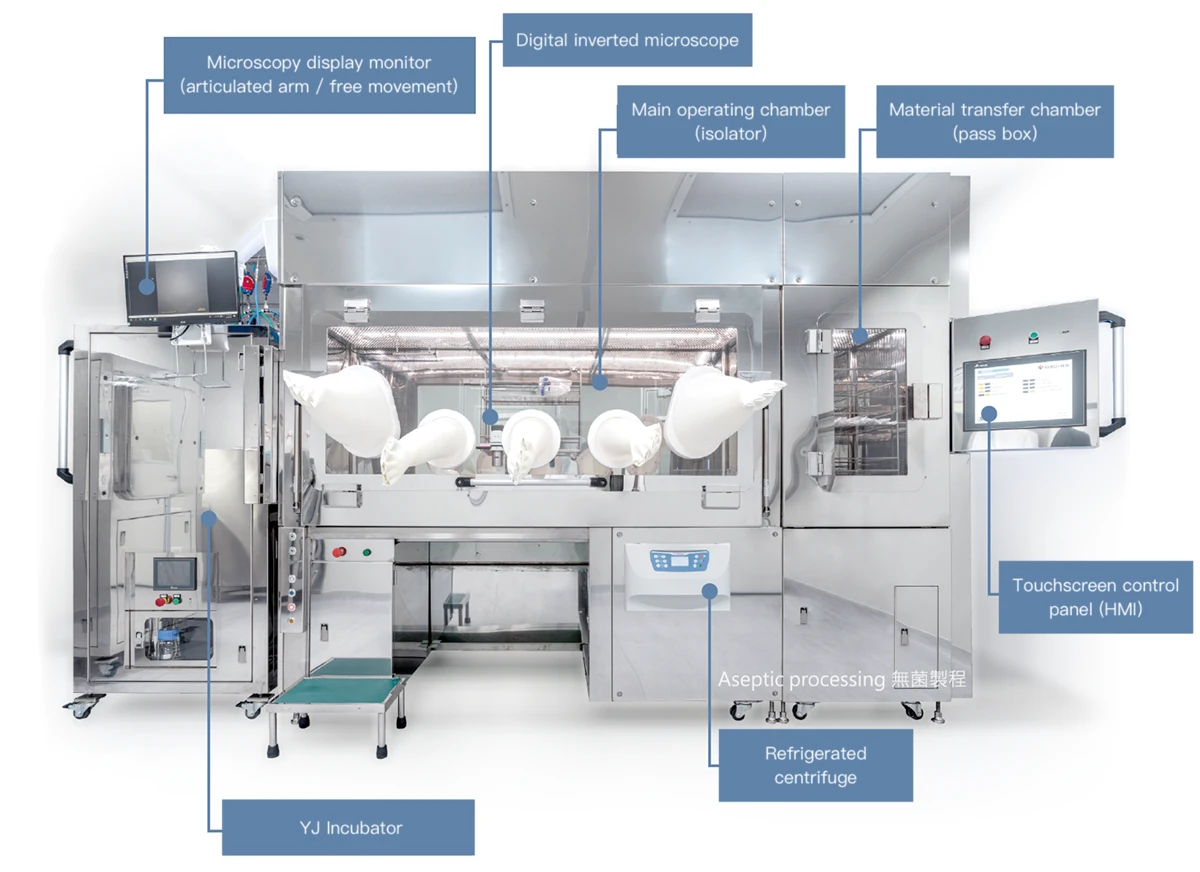
Exosome production is a multi-step operation that requires an ensemble of sophisticated equipment — covering various stages like cell culture, exosome harvest, filling, freeze-drying, and capping. These specialized equipment, when properly integrated, can markedly improve efficiency and quality control.
Below is an overview of the typical equipment in exosome manufacturing:
| Process Stage |
Typical Equipment |
| Aseptic Control & Integration |
YJ Isolator System + RTP Transfer + H₂O₂ Sterilization Module |
| Cell Expansion |
YJ Incubator + Isobox Module |
| Exosome Isolation & Purification |
Centrifuge, TFF, SEC systems |
| Quality Testing |
NTA, TEM, Western Blot, Flow Cytometry |
| Aseptic Filling |
YJ Aseptic Bio-Filling Line (YJ-52403) |
| Freeze-Drying |
YJ Shelf Lyophilizer (YJ-52404) |
| Vial Capping |
YJ Vial Capping Line (YJ-52405) |
When different equipment and workflow are suboptimal and all over the place, it may lead to process inefficiencies and increased cross-contamination. However, when essential equipment are put into isolator platform, and create an integrated solution that complies with international aseptic manufacturing standards.
Next, let’s take a closer look at one of the most critical production stages:
Sterility in freeze-drying — how to build a fully aseptic lyophilization setup.
How to Build a Fully Aseptic Lyophilization Setup

What Are the Contamination Risks During Freeze-Drying?
Freeze-drying is widely used to extend the shelf life of exosomes, yet contamination risks during this stage are often overlooked.
Freeze dryers themselves are designed to control temperature and pressure — but they often do not offer a sterile environment. Filling, drying, and capping operation in an open system will expose the product to airborne particulates, microbes, or pyrogens, a deviation from aseptic processing standards.
Manual handling also introduces major risks. Contaminants on gloves, gowns, or human skin can compromise cleanroom integrity, while operator movements can disrupt airflow and increase airborne particles.
How to Implement Aseptic Freeze-Drying? What Equipment Should Be Integrated?
To meet strict aseptic process requirements, YJ-Bio has developed a fully integrated system, combining the following key modules:
-
YJ Shelf Lyophilizer (YJ-52404)
⭢ Dual-chamber design with precise temperature control for efficient lyophilization.
-
Aseptic Bio-Filling Line (YJ-52403)
⭢ Automated filling in Class A environment, contamination control with H₂O₂ sterilization and RTP (Rapid Transfer Port) systems.
-
Vial Capping Line (YJ-52405)
⭢ Fully automated vial sealing, eliminating the need for manual capping in exposure.
These modules can be operated separately or be fully integrated into the YJ-Isolator platform, shielding the process in a closed, aseptic environment. This design ensures full PIC/S GMP compliance, while simultaneously addressing contamination control, workflow efficiency, and regulatory demand.
YJ-Isolator Platform as the Core for Aseptic Exosome Manufacturing

Why Choose an Isolator Over a Traditional Suite?
Many teams still rely on cleanrooms or biosafety cabinets (BSCs) for exosome manufacturing. But these traditional setups have clear limitations — high energy consumption, complex workflows, and difficulty in maintaining continuous sterility. These risks become even more critical during terminal operations like aseptic filling and lyophilization.
The YJ-Isolator redefines this approach. It functions as a self-contained micro cleanroom, maintaining Class A (ISO 5) conditions within an ISO 8 background, without requiring large-scale cleanroom construction or centralized HVAC systems.
This markedly reduces both installation and operational costs, while making it easier to pass GMP audits and comply with global standards.
What Equipment Can Be Integrated with the Isolator?
Based on client requirements, the YJ-Isolator can be modularly integrated with multiple pharmaceutical-grade process units, creating a fully enclosed aseptic production line:
-
Aseptic Bio-Filling Line (YJ-52403)
⭢ Performs filling in a Class A environment, integrated with H₂O₂ sterilization and RTP transfer ports.
-
Shelf Lyophilizer (YJ-52404)
⭢ Freeze-drying is initiated and monitored entirely within the isolator, with no lid-opening required.
-
Vial Capping Line (YJ-52405)
⭢ Fully automated vial sealing, eliminating the need for re-entry into open areas.
These systems are connected via RTP (alpha-beta transfer ports), ensuring a fully closed aseptic process — from filling to freeze-drying to capping — in full compliance with GMP aseptic standards and the principles of Contamination Control Strategy (CCS).
Real-World Simulation: How to Produce 16,000 Vials of Exosomes Per Month?
When planning equipment layout, a common question is:
“How well does this production scale?”
Here we demonstrate with different scenarios where the scalability and flexibility of the YJ-Isolator system really shines.
Scenario:
- 1 main Isolator system + 2 Incubator modules
- Each incubator handles 2 production batches simultaneously
- Each batch yields 1 liter of concentrated exosomes, filled into vials containing 3 × 10¹¹ (300 billion) exosome particles per vial
4,000 vials/batch × 2 batches/month × 2 incubators =
~16,000 vials/month
This setup reaches small-to-mid-scale production capacity without increasing cleanroom footprint. As demand rises, capacity can be further scaled by adding more incubators or running additional production shifts.
The takeaway:
A well-designed isolator system can support different stages from small batch R&D to preclinical manufacturing, clinical-grade production
early-stage commercialization with compact infrastructure.
Are the Equipment in Your Facility up to Regulatory Demand?
What International Standards Must be Met?
When exosome-based products are intended for clinical use or international distribution, one of the questions raised is:
“Are your processes and equipment compliant with regulatory standards?”
It’s essential to ensure patient safety and avoid future litigation or operational risks.
Based on available technical documentation, the YJ-Isolator system is designed to meet multiple international aseptic manufacturing standards, including:
- ISO 13408: Aseptic processing of healthcare products, including isolator system qualification
- ISO 14644-1 / 3 / 7: Cleanroom classifications, testing protocols, and operational configurations
- PIC/S GMP Annex 1 (2023 Edition): Contamination Control Strategy (CCS) requirements for sterile products
These standards clearly demand:
Key processes like freeze-drying, filling, and material transfer must go beyond manual handling — require engineered systems that ensure verifiable, closed, aseptic operations.
BSC vs. Isolator: What’s the Difference?
Production crew face a dilemma:
Should they use Biological Safety Cabinet (BSC) or invest in an isolator system?
Here’s a direct comparison based on YJ-Bio’s technical documentation:
| Criteria |
BSC (Biological Safety Cabinet) |
Isolator System |
| Fully Enclosed System? |
❌ No — open-front, exposed to air |
✅ Yes — sealed with pressure control |
| Sterilization Method |
Manual surface wiping |
Automated H₂O₂ sterilization |
| Cleanroom Requirements |
ISO 5 (Grade B) background needed |
Operates within ISO 8 (Grade D) |
| Operator Risk |
High — semi-open design, prone to error |
Low — glove ports isolate operator |
| Regulatory Alignment |
Partially meets GMP Annex 1 |
Fully complied with GMP CCS, ICH Q9/Q10 |
This comparison highlights a key insight:
While RABS or BSC-based systems may offer lower upfront cost, they fall short in compliance, contamination control, and production scalability in the long run. For teams aiming to meet GMP, CCS, and global regulatory demands
Conclusion: Start with Sterility — Build a High-Quality Exosome Equipment Ecosystem
Whether you’re developing exosome-based therapeutics, preparing for clinical trials, or scaling up for commercial manufacturing, the way you select and integrate your equipment will profoundly impact product quality, regulatory readiness, and operational cost.
The YJ-Isolator platform and its supporting modules are purpose-built to help teams build closed, verifiable, and sustainable aseptic processes — even within existing facility constraints. From filling, freeze-drying, and capping, to capacity simulation and regulatory alignment, this system delivers more than just hardware — it offers a real-world pathway to high-performance manufacturing.
Real competitiveness doesn’t come from just making the product — it comes from making it reliably, repeatedly, and in full compliance.
If you’re exploring your next move or seeking tailored advice for your specific application, we’d be happy to support you with deeper insights and custom solutions.
Solving the Problem at Its Source: YJ-Bio’s End-to-End Expertise
Now that you understand how high-performance exosome products are made, you may be wondering:
Where can I find a trustworthy exosome supplier?
The answer: Start from the source. Ensuring safety and efficacy begins with manufacturing.
We offer complete planning and setup services, including:
- Cleanroom layout and GMP zoning
- Isolator-based cell culture equipment integration
- DQ/IQ/OQ/PQ validation & VHP sterilization
- Full tech transfer and SOP implementation
Build a compliant, safe, and scalable production line — from the ground up.
🧪 YJ-Isolator: A GMP-Ready Workstation for Cell & EV Production
Purpose-built for cell and exosome manufacturing, our YJ-Isolator integrates:
- Incubators
- Microscopes
- Centrifuges
…and can be customized to fit your unique process flow. A complete GMP-grade production solution in one unit.
- Human umbilical cord-derived EVs (50 billion / 300 billion vesicles per batch)
- Immune and stem cell culture services for R&D and product development
Let us take care of the raw materials and upstream tech, so you can focus on delivering better products.
🔗 Further Reading & References
-
Manufacturing Therapeutic Exosomes: From Bench to Industry
A comprehensive overview of the full exosome production workflow and GMP compliance challenges.
-
Review on Strategies and Technologies for Exosome Production
Explores current methods for exosome isolation and scale-up technologies for GMP-grade manufacturing.
-
Freeze-Dried Extracellular Vesicles from Adipose-Derived Stem Cells
Research on lyophilization techniques and the role of cryoprotectants in exosome stability.
-
Aseptic Process Simulations for Lyophilisation: Challenges and Tips
Insights on contamination control strategies for freeze-drying, aligned with GMP Annex 1.
-
CRB Group – Aseptic Processing Insights
Industry perspectives on minimizing risks during aseptic operations, especially lyophilization.
-
Exosomes Produced in Bioreactors
Case study showcasing how bioreactor systems can significantly boost exosome yield.
-
Guidance on the Clinical Application of Extracellular Vesicles
Discusses sterility, contamination control, and regulatory guidance for clinical-grade exosome products.