The development of cell and gene therapies is progressing at an unprecedented pace, offering new treatment opportunities for diseases with high unmet medical needs. Among these, non-genetically modified cell therapies and their derived extracellular vesicles (EVs) have emerged as promising modalities due to their lower regulatory complexity and potential for earlier clinical adoption.
To ensure clinical efficacy and safety, manufacturing must be robust, scalable, and GMP-compliant. Traditional centralized manufacturing models, while established, present logistical challenges, including transportation risks and delays that may compromise cell viability or therapeutic potency. In contrast, point-of-care (POC) manufacturing, performed near or at the clinical site, reduces transportation risks, enables the delivery of fresh products, and accelerates treatment timelines.
Isolator-based systems offer a practical solution for POC manufacturing by maintaining fully sealed aseptic conditions. These systems allow critical processes—tissue acquisition, cell processing, gene modification, expansion, and final formulation—to be performed safely in a closed environment. Positive pressure isolators, in particular, protect sensitive cell and EV products from environmental contamination while supporting GMP compliance.
YJ Biotechnology’s study emphasizes the broad applications of immune cell-based therapies, such as CIK, NK, dendritic, and γδ T cells, as well as regenerative and stromal therapies like mesenchymal stromal cells (MSCs) and their derived EVs. These therapies can be autologous or allogeneic, genetically modified or unmodified, and require precise manufacturing controls to ensure safety, consistency, and potency.
The research highlights how Isolator-based POC manufacturing platforms provide modularity, scalability, and contamination control, making them ideal for hospital-based or decentralized production. By enabling on-site GMP-compliant manufacturing, these systems facilitate rapid delivery of personalized and allogeneic therapies while reducing reliance on centralized CDMOs.
This perspective underscores YJ Biotechnology’s commitment to advancing translational medicine by providing practical, safe, and scalable manufacturing solutions for next-generation cell and EV-based therapeutics.
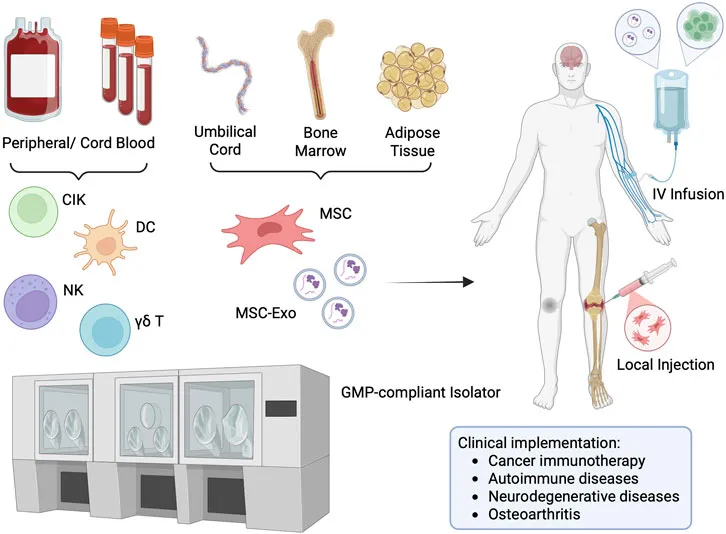
Figure 1. Isolator-Based POC Manufacturing for Cell/EV Therapies. Legend: Schematic representation of point-of-care manufacturing using an isolator system. Cells are first isolated from donor or patient tissue, then expanded and processed within a closed, GMP-compliant isolator in the hospital. This enables on-site production of immune or stem cell-based therapies and immediate administration back to patients. The entire workflow—from tissue extraction, cell isolation, expansion, formulation, quality control, to final infusion—can be seamlessly integrated and completed within the hospital-based isolator system. This end-to-end, closed-system approach minimizes contamination risk, ensures regulatory compliance, and significantly reduces time between manufacturing and patient treatment.
Ref: https://www.frontiersin.org/journals/bioengineering-and-biotechnology/articles/10.3389/fbioe.2025.1644318/full